2.3. Shape changes
Enzymes are globular structures made up of wrapped protein chains. In the entangled cluster of molecular chains that constitute them, enzymes possess specific sites (Fig. 15), which are slits or cavities of very particular sizes and shapes, capable of rigorously selecting the access of external molecules by a lock-and-key mechanism. These active sites are where the chemical reactions promoted by the enzyme take place. The accessibility to the active sites can be regulated by structural changes of the enzyme caused by interactions with external molecules. This is how the thousands of enzymes in our body can be activated or deactivated on demand, depending on the needs of the organism.
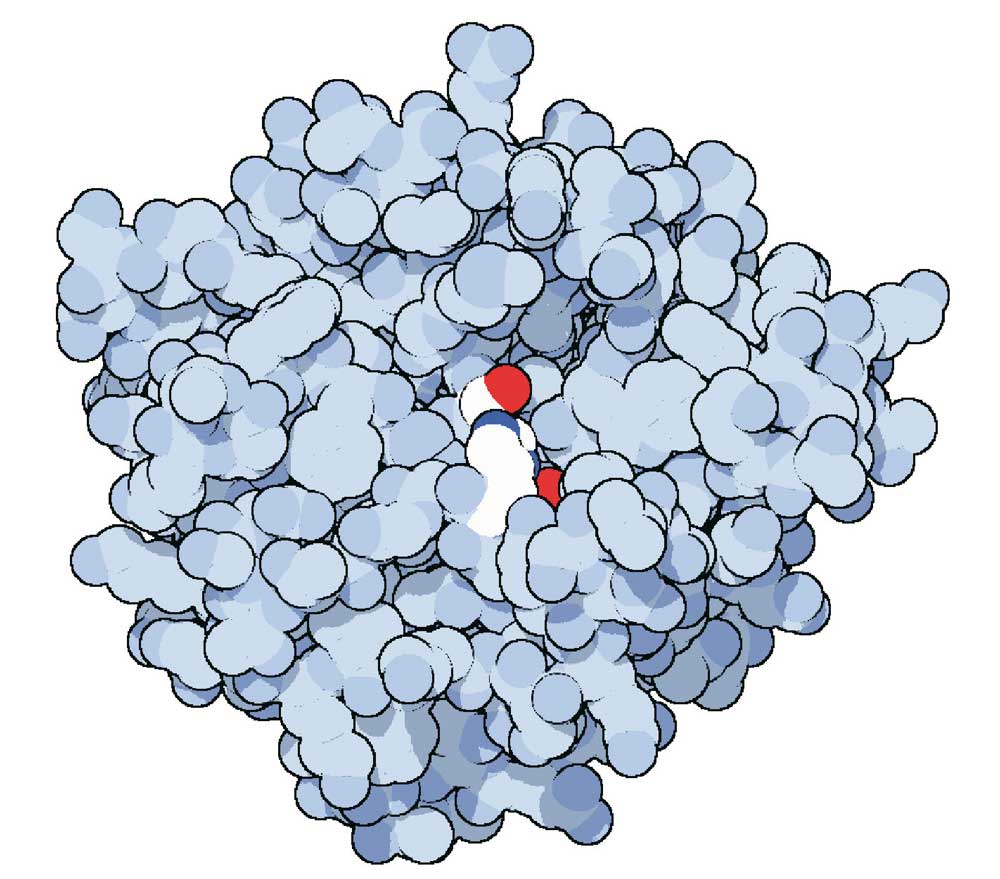
Fig. 15. Chymotrypsin is a very important enzyme for the functioning of our body. In this three-dimensional representation, the active site of the enzyme (in red) can be seen at the center of the cluster of protein chains (in gray). Credits: RCSC PDB and David S. Goodsell, The Scripps Research Institute, La Jolla, USA.
A typical example of an enzyme whose active site can be activated and deactivated is aspartate transcarbamylase, also known as ATCase. This enzyme is present in bacterial cells, where is responsible for the synthesis of two very important molecules, thymine and cytosine. As shown schematically in Fig. 16, ATCase is composed of six large catalytic units (that is, tasked with promoting the reaction) whose position is controlled by six smaller units, which constitute the regulatory system of the enzyme. Note that the representation shown in the figure is neither a chemical formula nor a scaled molecular model, but only a schematic drawing that attempts to explain the way this complex enzyme works.
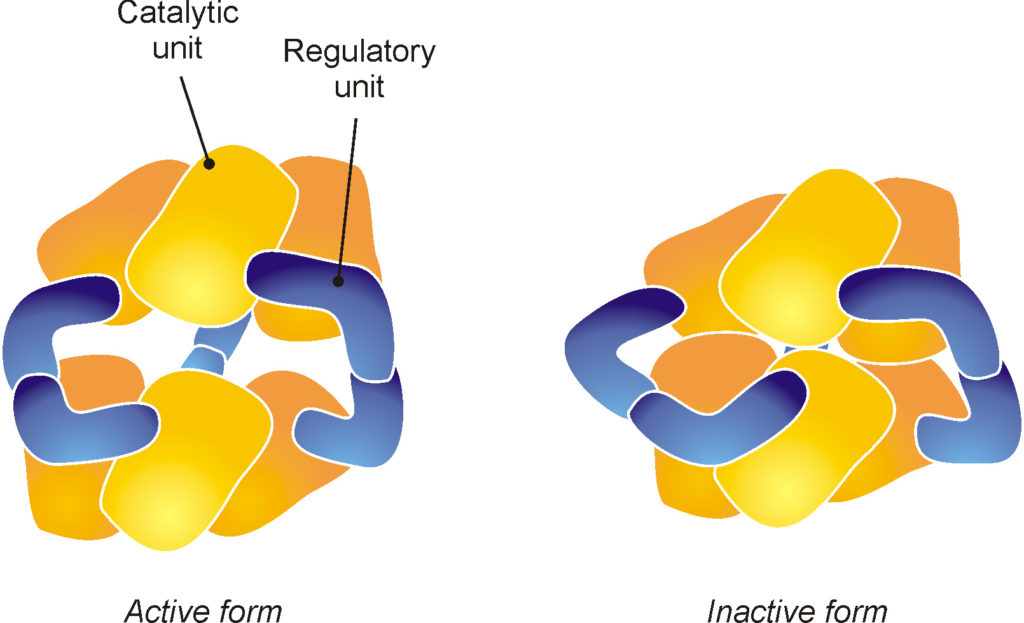
Fig. 16. The enzyme aspartate transcarbamylase is present in bacteria, where it is responsible for the synthesis of two important molecules, thymine and cytosine. This enzyme is able to self-regulate: an excess of synthesized molecules causes a change in shape (allosteric mechanism) that blocks the activity of the enzyme.
The active site of the enzyme is located where two catalytic units face one another; if they are slightly separated, their active sites are free and functional, while if they are in close contact, they interact directly, preventing the access of the reagents. When the molecules produced by the enzyme over accumulate, their presence causes the regulatory units to change shape, forcing the catalytic units to get closer until the active site is shut down.
Enzymes such as the one just described, whose operation relies on a change of shape, are called allosteric (from the Greek ἄλλοσ, different, and στερεóσ, solid). Allosteric effects are a very effective way devised by nature to provide feedback to the system in order to keep a chemical process under control.