3.4. Linear movements
Most research in the field of artificial molecular machines capable of performing linear movements is currently focused on systems called rotaxanes (Fig. 19). A rotaxane is formed by a filiform molecule threaded in a ring-like molecule; the presence of bulky groups (called stoppers) at the ends of the filiform component prevents the slipping off of the ring (Sauvage 1999)1Sauvage, J.-P., Dietrich-Buchecker, C., eds. (1999) Molecular catenanes, rotaxanes and knots, Weinheim: Wiley-VCH.. Systems of this kind, if carefully designed, can perform mechanical movements such as those shown in the figure when they are appropriately stimulated. The peculiarity of a rotaxane is that its molecular components, although not chemically bound together, cannot be dissociated. There is a mechanical constraint between wire and ring that maintains the integrity of the nanostructure, while allowing a certain degree of freedom of movement of the components with respect to one another (Bruns 2017)2Bruns, C. J., Stoddart, J. F. (2017) The nature of the mechanical bond: from molecules to machines, Hoboken: Wiley..
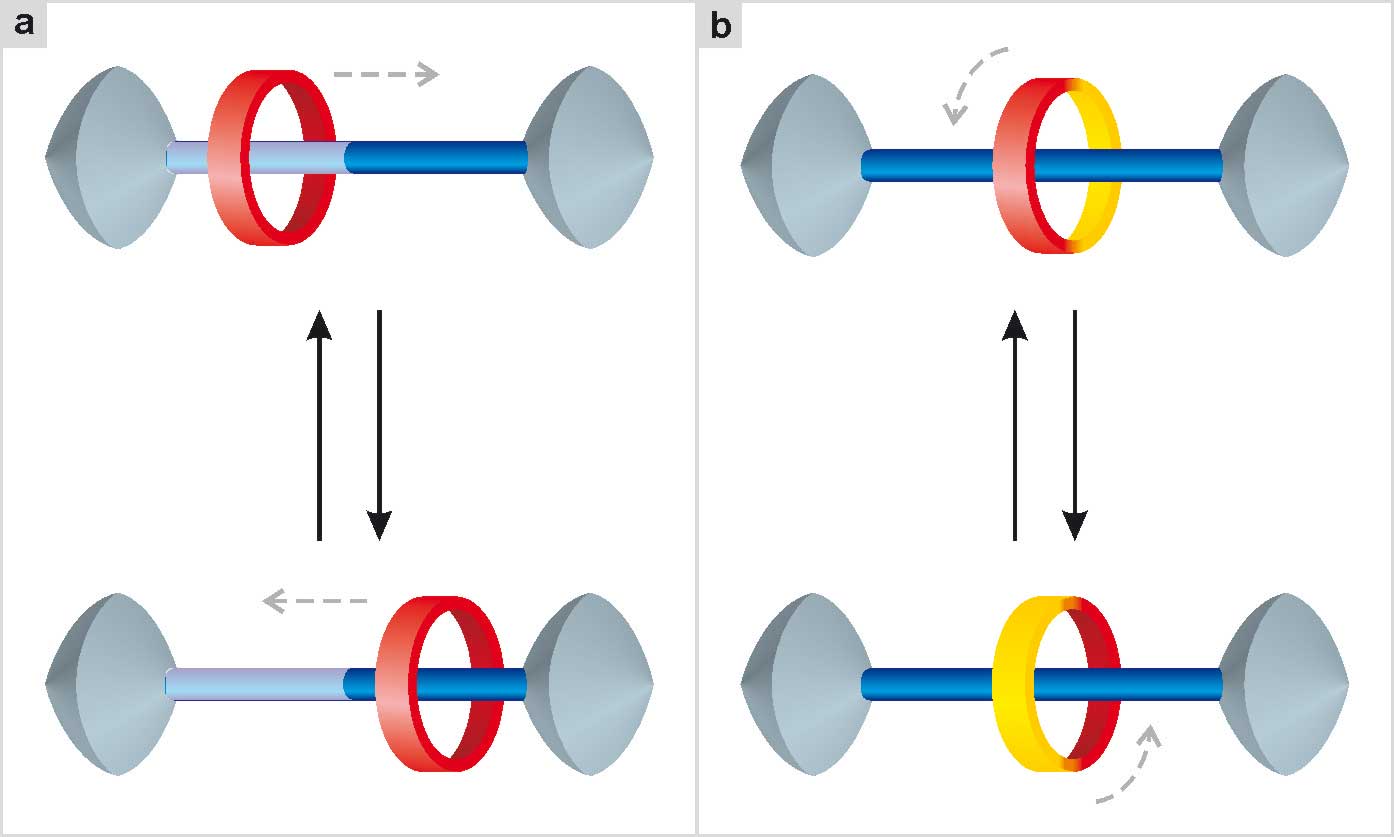
Below are some examples of molecular machines based on these systems, also chosen to show how light, chemical or electrical energy can be used to make mechanical movement happen.
Molecular shuttles
In a rotaxane, the movement of the ring along the thread (Fig. 19a) corresponds, at a molecular level, to the movement of a shuttle along a track. The first example of this type, developed in 1994 at the University of Birmingham (United Kingdom) by Fraser Stoddart’s group, is represented by the rotaxane 2 shown in Fig. 20 (Bissell 1994)3Bissell, R. A., Córdova, E., Kaifer, A. E. e Stoddart, J. F. (1994) A chemically and electrochemically switchable molecular shuttle, Nature, 369: 133-137.. It is formed by ring A and the linear component B in which there are two distinct units, B1 and B2. The first one, called benzidine, can be charged positively either by adding two protons (H+) or removing an electron. The second unit is called biphenol and, like benzidine, is an electron donor. These units represent two potential stations for the ring, which is instead an electron acceptor. It is attracted by both B1 and B2 by virtue of donor-acceptor interactions; however, since these interactions are stronger with B1 than with B2, the ring is initially on station B1. However, if an acid is added to the solution containing the rotaxane, the benzidine acquires two H+ ions and loses the ability to interact with the ring; consequently, the latter moves to station B2. If at this point a basic substance is added to the solution, the benzidine is restored and the ring returns to station B1, completing the operating cycle (Fig. 20b).
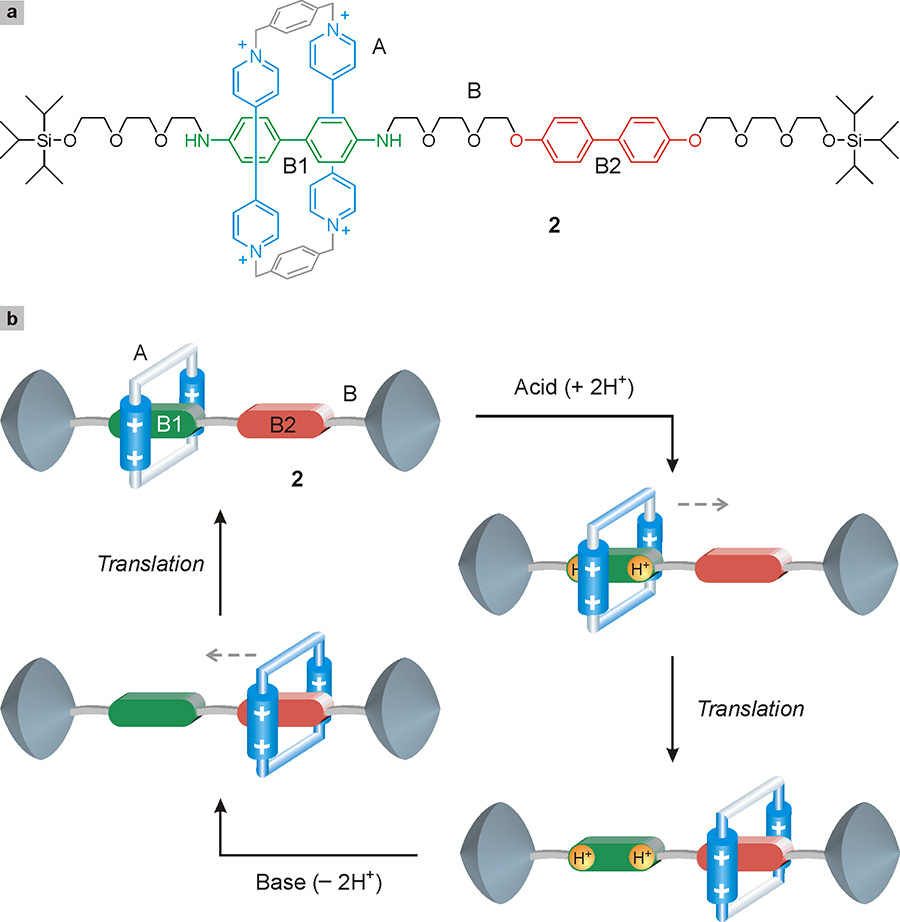
The alternating movement of A between B1 and B2 can be repeated many times because the acid-base reaction that governs it is perfectly reversible. The only limitation derives from the fact that subsequent additions of base and acid lead to the formation of waste products which, if not eliminated, after some time can compromise the functioning of the system.
The molecular shuttle shown in Fig. 20 can also be operated with electrical stimuli. In fact, removing an electron from the benzidine (chemists call this process oxidation) eliminates its ability to attract the ring, which then moves to B2. Subsequently, by returning the electron to the oxidized benzidine (reduction) it is possible to restore the primary station B1, which will again be surrounded by the molecular ring (Bissell 1994).
After this first study, numerous prototypes of molecular shuttles driven by chemical or electrical stimuli were described.
Examples of molecular light-driven shuttles are much rarer; one of them, developed in our laboratory in collaboration with the Stoddart group, is shown in Fig. 21 (Balzani 2006)4Balzani, V., Clemente-León, M., Credi, A., Ferrer, B., Venturi, M., Flood, A. H., Stoddart, J. F. (2006) Autonomous artificial nanomotor powered by sunlight, Proceedings of the National Academy of Sciences of the U.S.A., 103: 1178-1183.. The structural and functional complexity of this system gives an idea of the level of sophistication achieved in the design and construction of molecular machines. It is a rotaxane (3) comprising a ring component A, with characteristics of electron donor, and a linear component consisting of several modules: i) a ruthenium complex (R) which carries out, in addition to the function of stopper, also the fundamental one of absorbing the light used by the system; ii) two units, B1 and B2, having electron acceptor characteristics: these are the two stations on which ring A can stop; iii) a rigid spacer S and a second stopper T. The initial state of the system is that in which ring A surrounds unit B1, which is an electron acceptor more effective than B2. Following the light excitation of the ruthenium complex R, a series of movements take place in the system which can be described schematically as follows (Fig. 21).
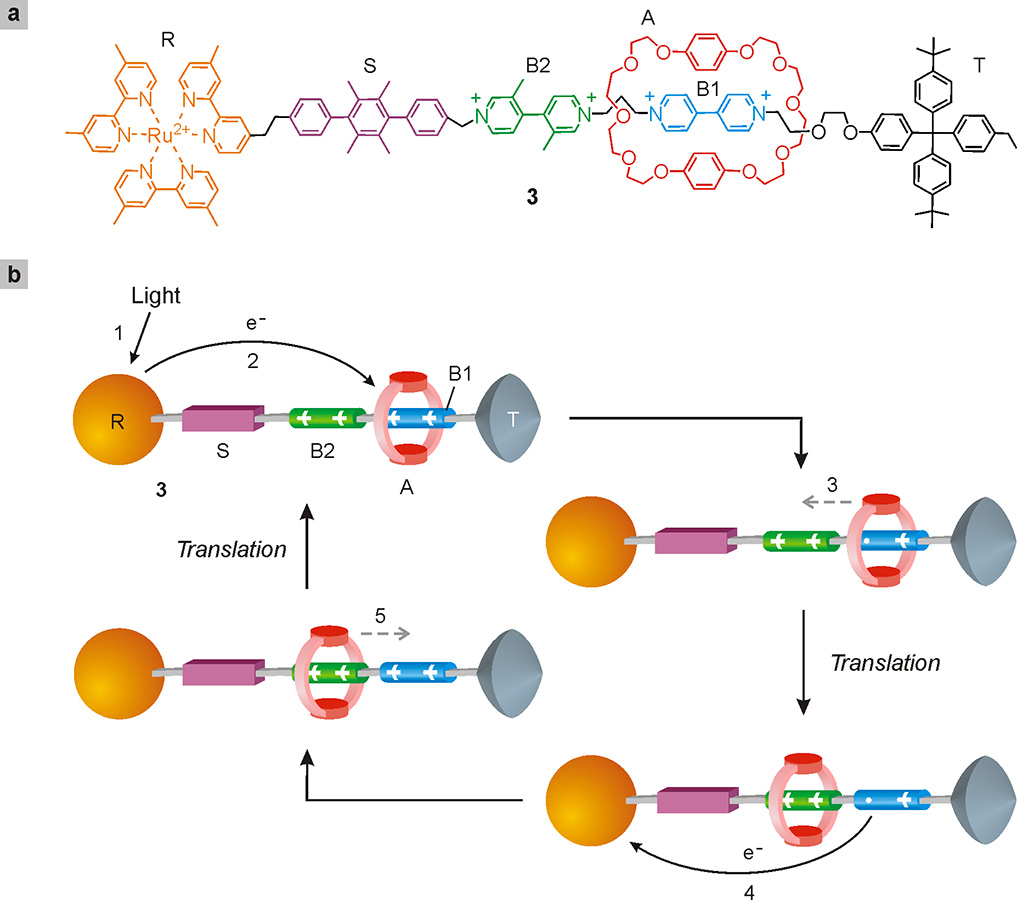
Fig. 21. A four-stroke linear nanomachine powered by light stimuli. Part (a) of the figure shows the simplified structure formula of rotaxane 3. In part (b), the sequence of events caused by light excitation is illustrated using a cartoon representation of the rotaxane. Initially, ring A resides on station B1. The absorption of a photon of light (process 1) by the ruthenium complex (R) is followed by the transfer of an electron (process 2) from R to B1. The latter unit no longer interacts with ring A, which consequently moves to B2 (process 3). At this point an electron returns from B1 to the ruthenium complex (process 4): the primary station is regenerated and the A ring returns onto it (process 5). All processes are very fast and an entire cycle takes place in less than one thousandth of a second.
a) Destabilization of the initial structure: following the absorption of light (process 1) an excited state of R is obtained which transfers an electron to the station B1 (process 2) surrounded by the ring A. Following this electron transfer, station B1 loses its electron acceptor characteristics and no longer interacts with A;
b) Displacement of the ring: as its interaction with B1 is lacking, ring A moves (process 3) and passes on station B2 with which it can interact;
c) Electronic reset: at this point a process opposite to that caused by light brings an electron from the disabled station B1 (no longer surrounded by A) back to the ruthenium complex that had initially transferred it (process 4), thereby restoring the electron acceptor character of station B1, which is thus reactivated;
d) Structural reset: following the electronic reset, ring A returns onto station B1 (process 5), restoring the initial structure.
In conclusion, a light pulse causes, through four stages, the alter-nate movement of the ring along the thread from right to left and then from left to right without generating waste products; this system can therefore be considered a four-stroke linear motor, operated by light.