3.5. Rotary movements
Catenanes
A catenane is a system formed by two (or more) ring molecules chained together; in it we find the mechanical constraint between the molecular components already seen for rotaxanes. The simplest catenane has only two rings (Fig. 24a). In specifically designed structures of this type, one ring can be made to rotate with respect to the other by means of appropriate stimulation. To highlight this movement, however, it is necessary that at least one of the two rings has different stations, as is the case of catenane 5 (Fig. 24b) developed by Sauvage and collaborators (Livoreil 1994)1Livoreil, A., Dietrich-Buchecker, C.O., Sauvage, J.-P. (1994) Electrochemically triggered swinging of a [2]-catenate, Journal of the American Chemical Society, 116: 9399-9400..
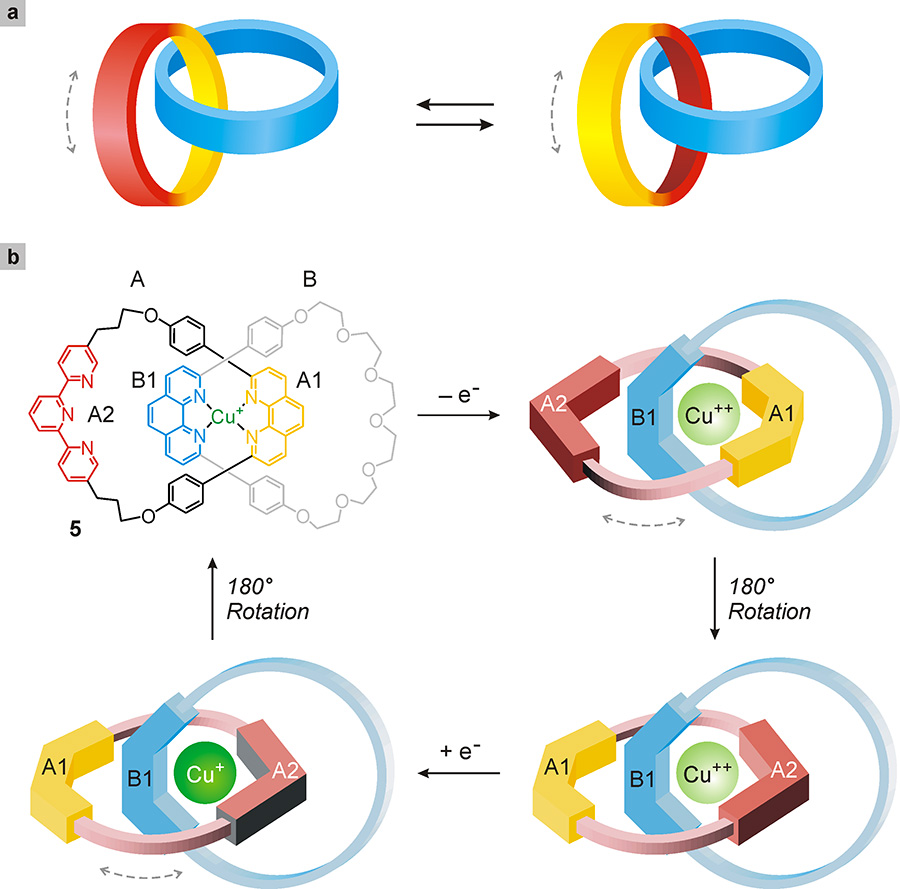
Fig. 24. (a) Schematic representation of the rotary movement of one molecular ring with respect to the other in a catenane. (b) Rotation of the rings of a catenane operated by electricity. Catenane 5 consists of two rings, A and B, which contain sites capable of binding a copper ion (Cu+). Initially, the Cu+ ion is bound to phenanthroline-type sites A1 and B1. The electrochemical oxidation of the copper ion to Cu++ causes the destabilization of the initial structure and the 180° rotation of the A ring with the formation of a structure in which the Cu++ ion interacts with the A2 and B1 sites. An inverse stimulus (reduction, which converts the Cu++ ion to Cu+) returns the system to the initial structure.
This catenane is constituted by ring A, which contains a phenanthroline (A1) and a terpyridine (A2) site, and ring B, which contains only a phenanthroline site (B1). The system also comprises a copper ion (Cu+), which binds strongly to the two phenanthroline sites (A1 and B1), forcing them to stay close together. To rotate the ring A with respect to B, this structure must be destabilized – a result that can be achieved with an electrochemical stimulus which, by removing an electron from the Cu+, ion, modifies its bonding properties. The Cu++ ion thus formed prefers to interact with the terpyridine site, causing a 180° rotation of ring A with respect to B.
If, at this point, again through an electrochemical stimulus, the electron that had previously been removed is returned to the copper ion, the latter regains its initial characteristics. Consequently, the ring A rotates again 180° with respect to B restoring the initial structure. Systems like the one just described, although very interesting, are limited by the fact that two successive 180° rotations will not necessarily take place in the same direction. In fact, assuming that the first rotation (induced by oxidation of Cu+ to Cu++) occurs in a clockwise direction, the subsequent rotation (induced by the reduction of Cu++ to Cu+) has the same probability of occurring clockwise or counterclockwise. In other words, catenanes such as 5 cannot function as rotary motors, instead behave like random oscillators.
Catenanes such as the one shown in Fig. 24 constitute however the starting point for obtaining the unidirectional and repeated rotation of a ring with respect to the other in response to external stimuli. A control element that allows to select the direction of rotation – clockwise or counterclockwise – in each movement of 180° needs to be included in the design. This was elegantly done at the University of Edinburgh by David Leigh and collaborators with the catenane 6 (Fig. 25) (Hernandez 2004)2Hernandez, J. V., Kay, E. R., Leigh, D. A. (2004) A reversible synthetic rotary molecular motor, Science, 306: 1532-1537.. It consists of a smaller ring A and a larger ring B; along the latter, the primary station B1, a bulky group T1, the secondary station B2 and a bulky group T2 are positioned in this order.
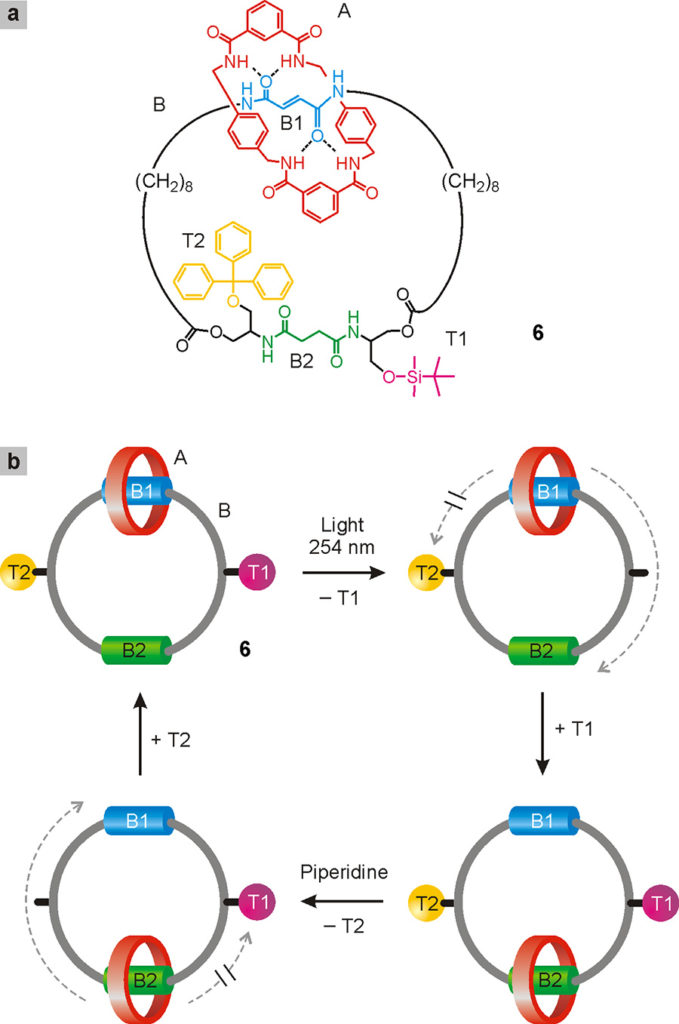
Initially, ring A surrounds station B1; when the latter is deactivated with a light stimulus, A tries to reach the B2 station. This movement, however, is only possible by removing one of the two bulky groups. For example, by selectively removing T1 as shown in the figure, ring A reaches B2 moving along B only in a clockwise direction, and there it remains trapped when T1 is repositioned. At this point, upon resetting station B1 by heating and selectively removing T2, ring A returns to its initial position moving along B in a clockwise direction. A system of this kind allows a total control of the relative motion of the two rings (unidirectional rotation in a clockwise or counterclockwise direction, or oscillation) depending on the order in which the stimuli that influence station B1 and bulky groups T1 and T2 are administered. The flip side of the coin is that the chemical transformations depicted in Fig. 25 are rather complex to carry out from a practical point of view. Subsequently, the same research group created a catenane in which the unidirectional rotation is driven by a single chemical reagent (Wilson 2016)3Wilson, M. R., Solà, J., Carlone, A., Goldup, S. M., Lebrasseur, N., Leigh, D. A. (2016) An autonomous chemically fuelled small-molecule motor, Nature, 534: 235-240..