4.3. Nanovalves: drugs become smart
To maximize therapeutic efficacy and minimize side effects, a drug should ideally react in a targeted manner within the body only where and when it is needed. Unfortunately, the reality is that drugs do not behave in this way as they often fail to reach the goal, possibly because they are degraded by the immune system or attack healthy tissues. In some cases, the active ingredient in the drug remains in the body for too long, causing adverse reactions. In other cases, drugs can remain in circulation for too short a time to be effective. One way to overcome these drawbacks is to use controlled release systems (drug delivery). They are essentially compounds, or groups of compounds, able to host the drug molecule, transport it into the organism and release it in the right place and at the right rate. The development of efficient, versatile and selective systems for controlled transport and release is undoubtedly one of the main topics of pharmacological research.
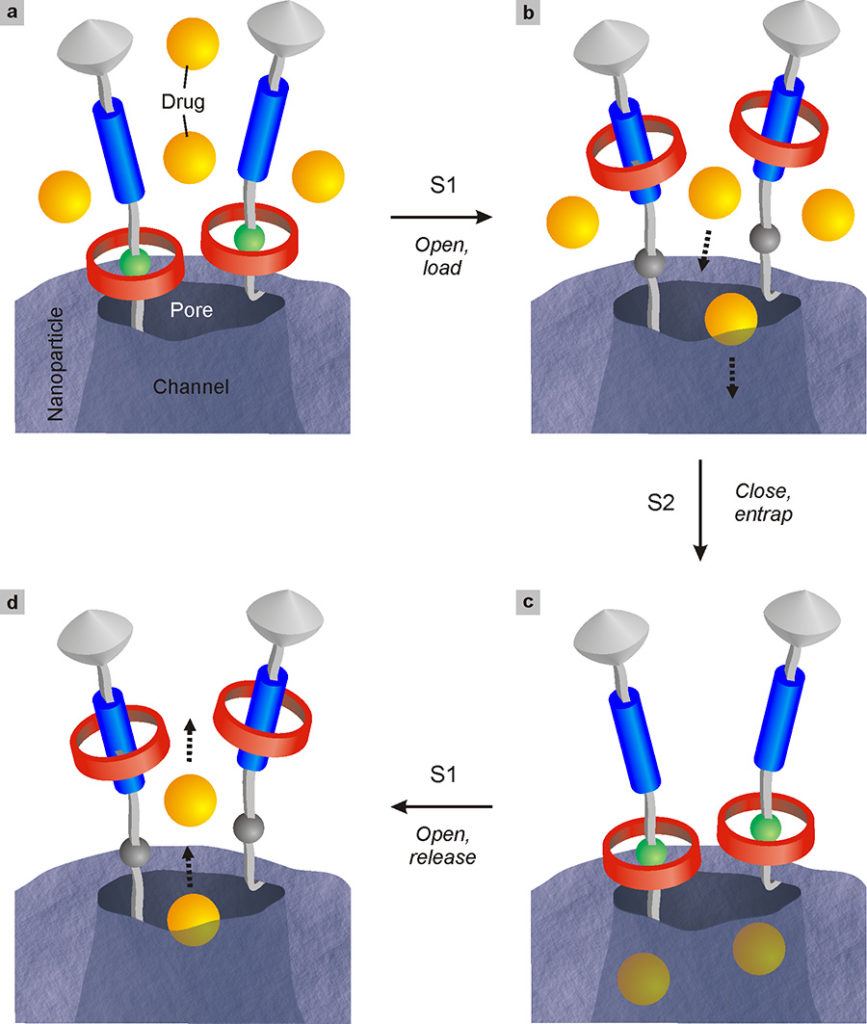
Porous silica nanoparticles are interesting in this context because they possess channels in their interior that can host small molecules; they are also stable, biocompatible, non-toxic and easy to prepare. To carry out the controlled release, however, it is necessary to have a strategy that allows to trap the drug in the nanoparticle and to re-lease it by an action triggered by a stimulus that can be endogenous (for example a tumor marker) or external (for example the light). This result has been obtained by exploiting the movement of molecular machines to open and close the entrance of the pores that connect the internal channels with the surface of the nanoparticle (Bruns 2017).
As shown in Fig. 28, molecular shuttles similar to those described in Fig. 20 have been chemically bonded to the surface of the nanoparticles, near the pores. Experiments have shown that the pores can be opened and closed by moving the rings of the rotaxanes far and near with respect to the entrance of the pores, respectively. The nanoparticle functionalized with molecular machines therefore behaves as a kind of nanometric valve (Fig. 28). The properties of these nanovalves can be adjusted by modifying structural parameters such as the length of the connector between the rotaxane and the surface, the distance between the stations of the shuttle, and the initial position of the movable ring. Through this approach, nanovalves have been constructed that are controlled by light, enzymatic or ionic stimuli (e.g. pH variations), capable of accommodating and releasing various types of molecules, including metal complexes, fluorescent species and anticancer drugs. Although we are still very far from Asimov’s Fantastic Voyage, these results give an idea of the potential offered by molecular machines in the medical field.